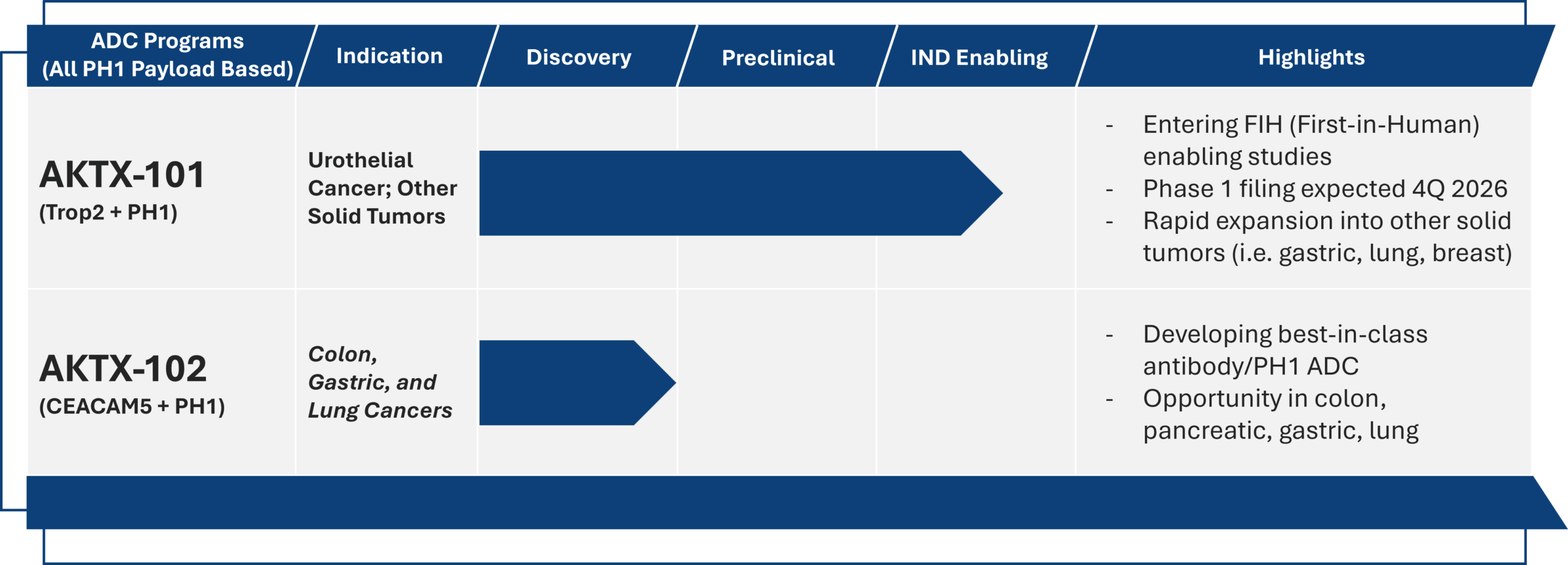
Akari Is Advancing PH1 Payload In Multiple ADC Programs
PH1 payload is the cornerstone of building an ADC pipeline against wide range of tumors
AN INNOVATIVE TARGETED ONCOLOGY COMPANY BUILT ON NEXT GENERATION ANTIBODY-DRUG CONJUGATES (ADC) AND A NOVEL DISCOVERY ENGINE