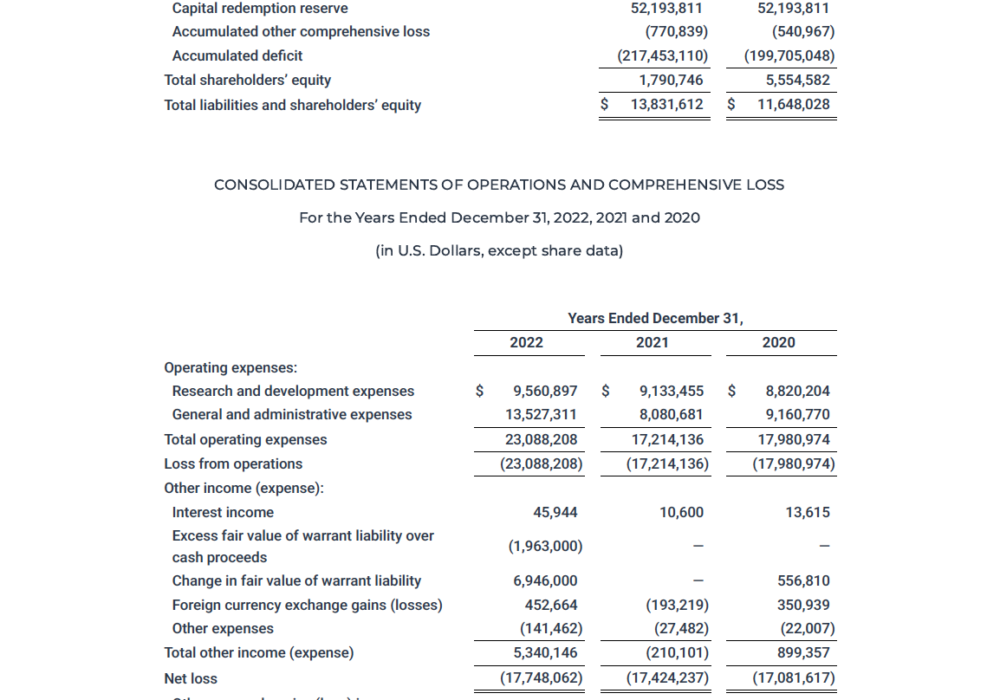
NEW YORK and LONDON, May 01, 2023 (GLOBE NEWSWIRE) -- Akari Therapeutics, Plc (Nasdaq: AKTX), a late-stage biotechnology company developing advanced therapies …
[Read more...] about Akari Therapeutics Reports Full-Year 2022 Financial Results and Highlights