NEW YORK and LONDON, September 13, 2019 – Akari Therapeutics, Plc (Nasdaq: AKTX), a biopharmaceutical company focused on innovative therapeutics to treat orphan …
Akari Therapeutics to Participate in Citi and H.C. Wainwright Conferences in Early September
NEW YORK and LONDON, September 3, 2019 – Akari Therapeutics, Plc (Nasdaq: AKTX), a biopharmaceutical company focused on innovative therapeutics to treat orphan …
Akari Therapeutics’ Nomacopan Granted U.S. Orphan Drug Designation for Hematopoietic Stem Cell Transplantation-Associated Thrombotic Microangiopathy (HSCT-TMA)
NEW YORK and LONDON, August 30, 2019 – Akari Therapeutics, Plc (Nasdaq: AKTX), a biopharmaceutical company focused on innovative therapeutics to treat orphan …
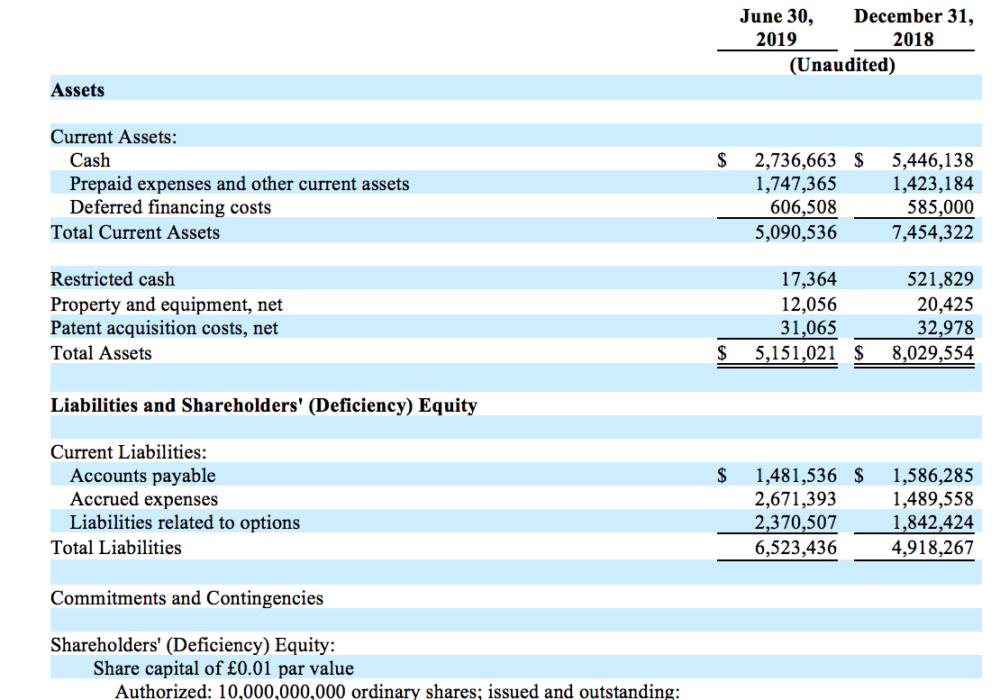
Akari Therapeutics Reports Second Quarter 2019 Financial Results And Highlights Recent Clinical Progress
Positive early safety and efficacy data with nomacopan Phase II clinical study in patients with mild-to-moderate bullous pemphigoid (BP) announced April …
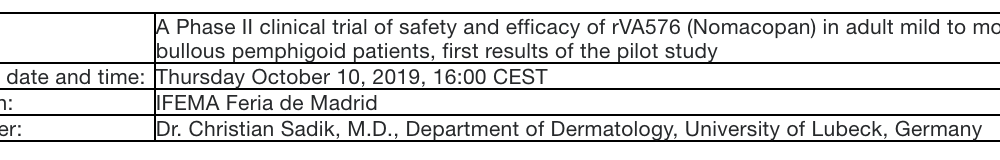
Akari Therapeutics Announces Oral Presentation of Nomacopan Phase II Data in Patients with Bullous Pemphigoid at the 28th European Academy of Dermatology and Venereology (EADV) Congress, October 9-13, 2019
NEW YORK and LONDON, July 31, 2019 (GLOBE NEWSWIRE) -- Akari Therapeutics, Plc (AKTX), a biopharmaceutical company focused on innovative therapeutics to treat …