1. Complement C5 inhibition efficacy
Nomacopan C5 inhibition supported by clinical PNH research
2. Simple, fixed dosing
Nomacopan clinical trials are establishing a simple, fixed dose in children; ease of dosing at home or in hospital for adults
3. Rapid onset & offset of action
Rapid onset/offset of action allows complement re-activation when needed
4. LTB4 inhibition may slow GVHD progression
LTB4 is often elevated in patients with GVHD and nomacopan inhibition of LTB4 may slow GVHD progression

A patient in the Phase 3 Part A clinical trial with severe pediatric HSCT-TMA, which typically involves multi-organ failure and other acute consequences, was discharged home from the hospital following treatment with nomacopan. The case study was presented as a late-breaker at Transplantation and Cellular Therapy Tandem Meetings and the European Society for Blood and Marrow Transplantation (EBMT) 49th Annual Meeting.
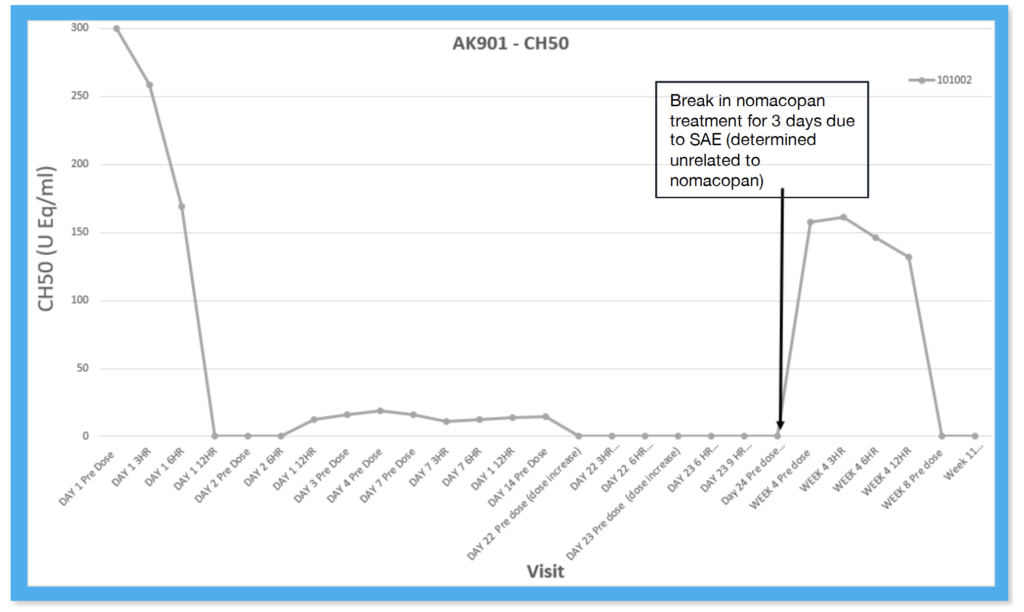
Clinical Response to Nomacopan in the Pediatric HSCT-TMA Setting presented Feb. 16, 2023, at the Transplantation & Cellular Therapy Tandem Meetings
- 6-year-old male received a cord blood HSCT for relapsed refractory acute myelogenous leukemia (AML)
- Post-transplant acute gut graft-versus-host disease (GVHD)
- TMA at day +66 post-transplant
- Treatment with a single-age, weight-based ablating dose of nomacopan day +74 followed by maintenance dosing for 21 days
- After a 3-day break in treatment for encephalopathy unrelated to nomacopan, treatment continued for a further 46 days until the end of the study with correction of the patient’s urine protein creatinine ratio for ≥28 days
- Gut pathology and thrombocytopenia resolved
- No adverse events related to nomacopan


