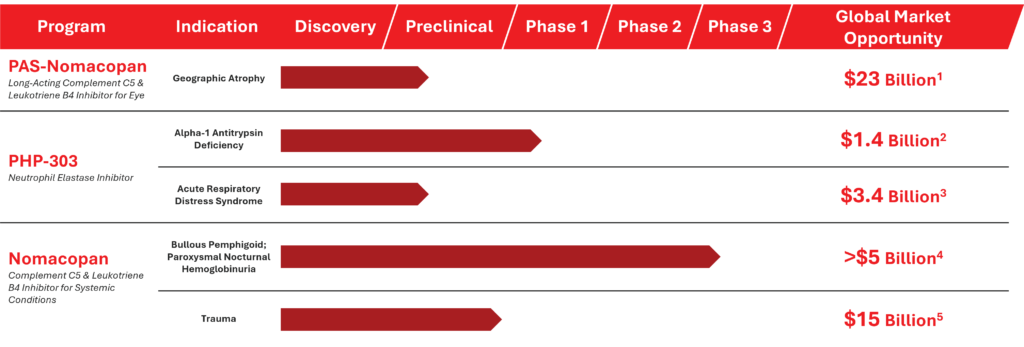
Assets Beyond ADC Platform
Akari Therapeutics is a biotechnology company developing next-generation precision bi-functional antibody drug conjugates (ADC) for the treatment of cancer. Beyond our priority ADC pipeline, we have a pipeline of legacy assets that are not currently being developed with potential across a number of high value indications. These programs are available for partnering and have the potential to bring in non-dilutive capital into the Company.